The O’Halloran group identified many of examples of metalloregulatory proteins–factors that govern metal responsive gene expression. Through structure, function, genetic and thermodynamic studies the group has established the molecular basis of allosteric control and metal ion selectivity. This work revealed that the pool of free zinc or copper ions in the cellular cytoplasm under resting conditions is vanishingly small, challenging long-held views that Fe, Cu and Zn are trace elements in cells. In 2001 Caryn Outten was the first to analytically define the cellular metallome (Figure 1) and showed that the total content of essential transition metals is tightly controlled in the millimolar range.
The O’Halloran group also discovered a new family of intracellular metal trafficking proteins called metallochaperone proteins. The group defined the structures, functions and mechanisms of a number of these proteins that control intracellular distribution of copper. These freely diffusing proteins traffic copper to specific cytosolic targets, and have since been found in many kingdoms of life.
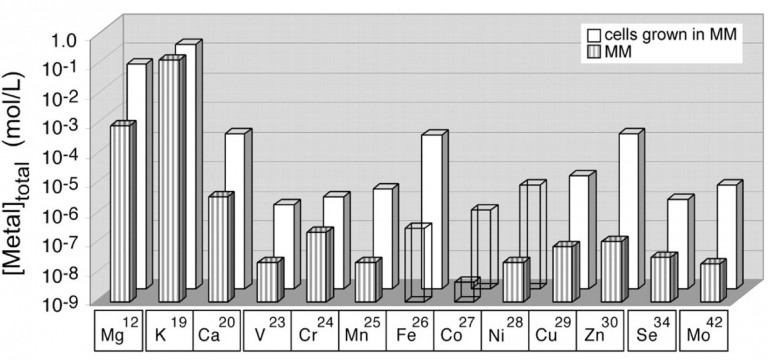
Figure 1. The E. coli Metallome. The total metal content of E. coli grown in minimal media (grey) as compared with the total metal content of media alone (white) determined by inductively coupled mass spectrometry (ICP-MS) (Outten et al., 2001).
Selected Publications:
- Gilston BA, O’Halloran T. “Mechanisms Controlling the Cellular Metal Economy”. In: Cullota V, Scott R, editors. The Encyclopedia of Inorganic and Bioinorganic Chemistry: Metals in Cells. Chichester, UK: John Wiley & Sons, Ltd.; 2013. p. 3-14.
- Outten CE, O’Halloran TV. “Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis”. Science. 2001;292(5526):2488-92.
- Lin, S., Pufahl, R., Dancis, A., O’Halloran, T.V., Culotta, V.C. “A Role for the Saccharomyces cerevisiae ATXI Gene in Copper Trafficking and Iron Transport.” J. Biological Chemistry. 1997; 272:9215-9220.
- Pufahl RA, Singer CP, Peariso KL, Lin SJ, Schmidt PJ, Fahrni CJ, Culotta VC, Penner-Hahn JE, O’Halloran TV. “Metal ion chaperone function of the soluble Cu(I) receptor Atx1”. Science. 1997;278(5339):853-6.
- O’Halloran, T. V. “Transition Metals in Control of Gene Expression.” Science.1993;261:715-725.

