Transition metals play a critical role in the regulation of cell differentiation and division. Members of the O’Halloran group, along with collaborator Teresa Woodruff, were the first to characterize the regulatory role of zinc fluxes during meiosis (the process by which all sexually reproducing eukaryotes produce mature gametes) and fertilization. Both the mammalian egg and sperm must progress through multiple maturation or activation events in order to successfully fuse and form a healthy zygote. Using a combination of Confocal (ZincBY-1), electron (STEM-EDS) and X-ray Fluroscence Microscopy (XFM) the group demonstrated that the GV oocyte accumulates approximately 20 billion zinc atoms during maturation. Following fertilization, however, the newly formed embryo rapidly loses approximately 12 billion zinc atoms. This rapid efflux of zinc is termed the ‘zinc spark’. Further studies established that zinc sparks are absolutely necessary for the production of a viable mouse embryo.
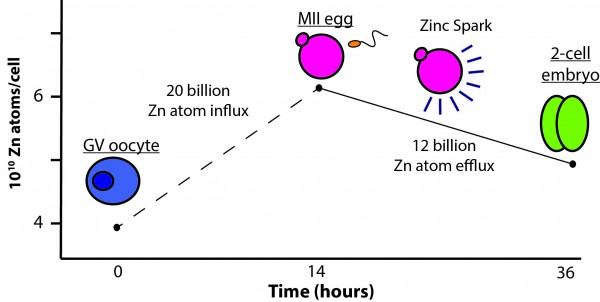
Figure 1: Zinc Fluxes During Meiosis and Fertilization Gametogenesis progression is paired with the quantitative zinc fluxes observed at each stage in development. Oocytes (blue) show an intact germinal vesicle (GV). Mature eggs (pink) have a visible first polar body. (Allison Kim, 2010)

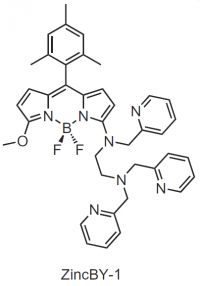
Figure 2: (Left) Tomographic images of Zinc Fluoresence X-ray fluorescence microscopy (XFM) tomography imaging of a sulfide-treated MII egg (left) shows an identical hemispherical, cortical distribution of zinc. Live cell fluorescence imaging (center) of ZincBY-1 (50 nM, green) and Hoecsht (1 µg/mL, blue) stained MII eggs. (Right) Structure of Zinc-BY-1, an ultra-senstive zinc fluorophore developed by Emily Que to probe zinc fluxes in live oocytes.
The Zinc Spark Mechanism
The O’Halloran group is interested in probing the mechanisms that drive these zinc sparks. To accomplish this, the group developed a new sensitive zinc-sensing fluorescent dye, ZincBY-1, capable of visualizing unbound zinc within a live oocyte without disrupting normal zinc homeostasis. Using this new zinc probe, the group demonstrated that prior to fertilization, zinc is stored in thousands of vesicles lining the periphery of the egg in a hemispherical pattern opposite of the meiotic spindle. Following fertilization, zinc is rapidly released in a series of localized bursts as shown in Figure 3. These new visualization tools will enable the group to better understand the molecular targets that control the observed zinc uptake, release, and redistribution within the oocyte and egg during meiotic maturation and fertilization with the goal to leverage these findings to improve IVF outcomes.
Figure 3: Timelapse of Zinc Sparks Mouse eggs release zinc into the extracellular environment through short but intense “sparks” upon activation by strontium chloride. The pattern of zinc release is unique to each egg. Note the polarized distribution of zinc release. This short video loop is excerpted from a longer time-lapse series. (Kim, et al. ACS Biology 2011)
Selected Publications:
- Duncan FE, Que EL, Zhang N, Feinberg EC, O’Halloran TV, Woodruff TK. “The zinc spark is an inorganic signature of human egg activation” Scientific Reports. 2016 Apr 26;6:24737.
- Zhang N, Duncan FE, Que EL, O’Halloran TV, Woodruff TK. “The fertilization-induced zinc spark is a novel biomarker of mouse embryo quality and early development” Scientific Reports. 2016 March 18;6:22772.
- Kong BY, Duncan FE, Que EL, Xu Y, Vogt S,O’Halloran TV, Woodruff TK. “The inorganic anatomy of the mammalian preimplantation embryo and the requirement of zinc during the first mitotic divisions.” Dev Dyn. 2015 Aug;244(8):935-47. Epub 2015 Jul 16.
- Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, Chen S, Garwin SA, Bayer AR, Dravid VP, Woodruff TK, O’Halloran TV. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nature Chemistry. 2014;7(2):130-9.
- Kong BY, Bernhardt ML, Kim AM, O’Halloran TV, Woodruff TK. Zinc Maintains Prophase Arrest in Mouse Oocytes Through Regulation of the MOS-MAPK Pathway.Biol Reprod. 2012 Jul 1;87(1):11.
- Kim AM, Bernhardt ML, Kong BY, Ahn RW, vogt S, Woodruff TK, O’Halloran TV. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol. 2011 Jul 15;6(7):716-23 Epub 2011 Apr
- Kim AM, Vogt S, O’Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Bio. 2010 Sep;6(9):674-81. Epub 2010 Aug 8.



